Definition and Examples of Analogy in Literature. Protons are made up of smaller.

Labeled Parts Of An Atom Diagram Atom Diagram Atom Worksheets
An atom is the basic unit of a chemical element.
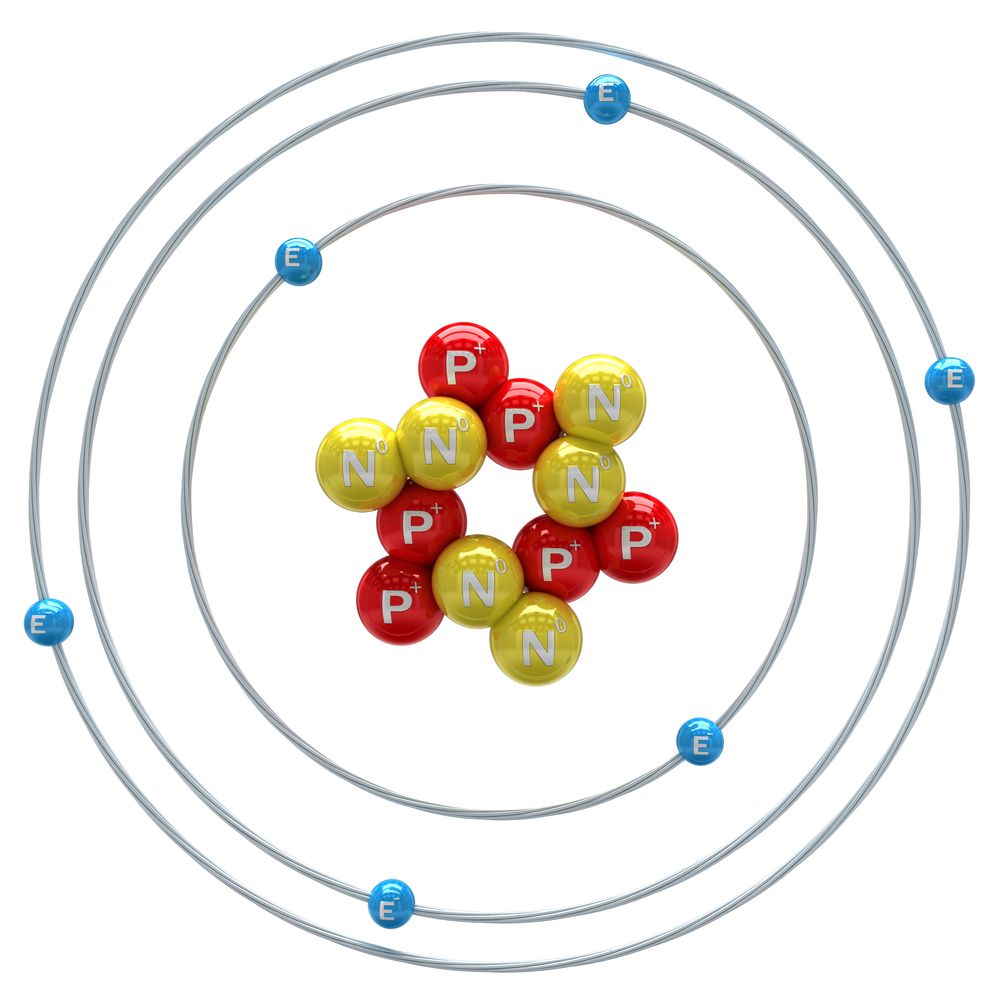
. Answer 1 of 8. Protons neutrons and electrons. 112 describe the structure of an atom as a central positively charged nucleus containing protons and neutrons most of the mass surrounded by orbiting electrons in shells.
Protons neutrons and electrons. The programme is equally relevant to design and technology engineering and as a STEM enrichment activity. An analogy is a comparison between two objects or systems of objects that highlights respects in which they are thought to be similarAnalogical reasoning is any type of thinking that relies upon an analogy.
Up to 24 cash back Use an analogy to describe the size or composition of the atom. Everything in the world is made out of atoms. Topics The structure of the atom intra-atomic forces the the atom is a tiny solar system comparison.
The slideshow runs on a continuous loop in the classroom 7th Grade Georgia while the students create cell analogies of their own. Molecules and compounds consist of atoms but are not themselves atomsExamples of molecules and compounds. The solar system analogy has a number of misalignments to the structure of the atom such as electrons being repelled rather than attracted by each other and that electrons do not have individual orbits like planets but have orbit clouds of electron density.
An analogical argument is an explicit representation of a form of analogical reasoning that cites accepted similarities between two systems to support. An example would be comparing the structure of an atom as a solar system with the nucleus. Rationale Teachers and textbooks often use analogies to introduce unfamiliar ideas.
It took until the twentieth century however for scientists to invent instruments that permitted them to probe inside an atom and find that it is not as had been thought hard and indivisible. Metaphor is used to relate the nucleus to the sun and the electrons to the planets without using the words like or as. Composition of the Atom.
Given an atomic number Z and mass number A you can find the number of protons neutrons and electrons in a neutral atom. Thomsons atomic structure described atoms as electrically neutral ie. Instead the atom is a complex structure composed of.
The atom is made up of smaller particles called subatomic particles Some subatomic particles are made up of even smaller particles Figure 232. Analogy plays a significant role in problem solving as well as decision making argumentation perception generalization memory creativity invention prediction emotion explanation conceptualization and communication. Some matter is either smaller or larger than an atomExamples of chemical species that are not typically considered atoms includes particles that are components of atoms.
This is a slideshow I created as an example of an analogy one could use to explain the function of organelles in a cell. Instead of writing their actual masses in kilograms we often use their relative masses. The Schroedinger Wave Equation describes the amplitude and other characteristocs of the waves which are associated with the moving electrons and thus it also is able to describe the energy and location of the orbiting electrons.
Protons and neutrons have appr View the full answer. One analogy is to imagine the atom is the size of a football stadium. For example a lithium atom Z3 A7 amu contains three protons found from Z three electrons as the number of protons is equal to the number of electrons in an atom and four neutrons 7 3 4.
The nucleus may also contain neutrons which have virtually the. The nucleus which is in the center of the atom and contains protons and neutrons and the outer region of the atom which holds its electrons in orbit around the nucleus. Shes as blind as a bat You have to be as busy as a bee to get good grades in high school Finding that lost dog will be like finding a needle in a haystack.
One classical atom model is the solar system model which was proposed by Bohr. Fixperts is an award-winning hands-on learning programme that challenges young people to use their imagination and skills to create ingenious solutions to everyday problems. Atoms are made of three main parts.
Solution A An atom is composed of two regions. In the case of introducing atomic. So one needs to know the context in which you are trying to explain the role and nature of electrons.
What Are Not Atoms. Here an atomic structure is compared to a solar system by using the word like. It is commonly referred to as the plum pudding model because it can be visualized as a plum pudding dish where the pudding describes the positively charged atom and the plum pieces describe the electrons.
The idea that matter is composed of tiny particles called atoms is at least 25 centuries old. The nucleus contains protons which have a positive charge equal in magnitude to the electrons negative charge. Hence similes and metaphors are employed to develop an analogy.
Range who have already studied the topic of atomic structure. For example a gold coin is simply a very large number of gold atoms molded into the shape of a coin with small amounts of other contaminating elements. An atom is the smallest unit of matter that retains all of the chemical properties of an element.
An analogy is required when introducing a new topic or idea to a person in terms that he is familiar with. If this were the volume of the atom. Structures Trends Chemical Reactions Quantitative Chemistry and Analysis.
The atom consists of a tiny nucleus surrounded by moving electrons. Therefore it is a simile. The positive and the negative charges were of equal magnitude.
Comparing two objects or ideas is common practice in the English language as useful in writing and literature as in. This is analogous to the wave mechanical model which visualizes the atom as a positive nucleus surrounded by vibrating electron waves. An analogy is a comparison of two things usually for explanation or clarification.
One analogy that is commonly used is that the atom is like a tiny solar system. Gold atoms cannot be broken down into anything smaller while still retaining the properties. But it is still a good analogy to the dimension of an atom.
What does the existence of a nuclear force explain. In that gigantic stadium the nucleus would only be the size of a small marble sitting on the 50-yard line. The structure of a carbon atom not drawn to scale The masses of subatomic particles are very tiny.
Rutherfords model of the atom modified by Niels Bohr made an analogy between the atom and the solar system. What is an analogy. Atomic theory continues to develop.
Basically the atom is as void as the solar system almost all the mass is accumulated to the nuclei 997. Although it was proved to be wrong because of the quantum mechanics. CCEA Double award science.

A Simple Representation Of The Sodium Atom Download Scientific Diagram
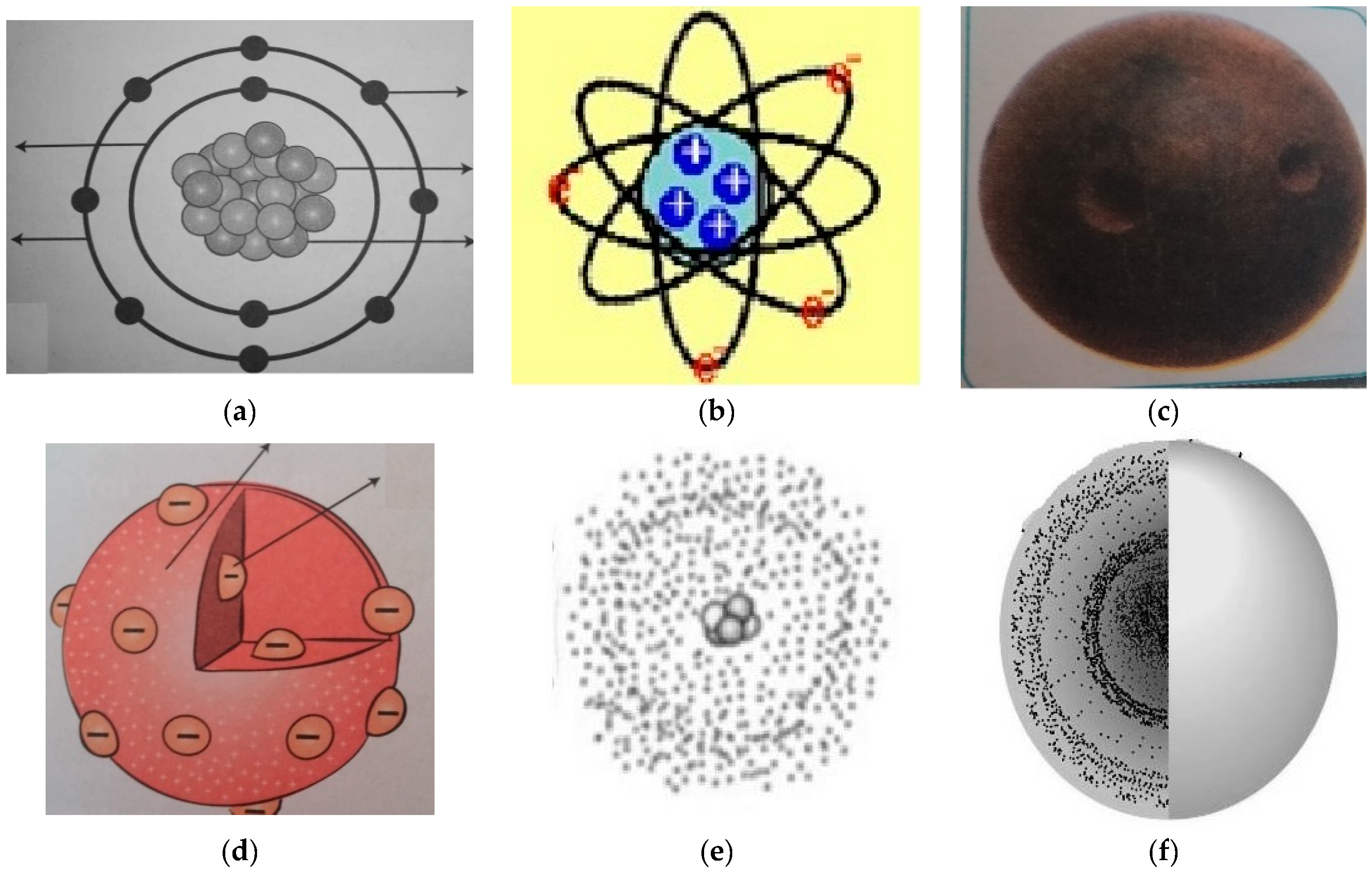
Education Sciences Free Full Text Insights Into Components Of Prospective Science Teachers Mental Models And Their Preferred Visual Representations Of Atoms Html
0 Comments